Figure 1: Acetic Acid Phase Diagram – Violation of Transitivity
Many high-school chemistry texts, general science texts, and similar authorities emphasize the distinction between “chemical” change and “physical” change. (Often the first lesson of the school year revolves around this distinction.) There is one major problem with this, and a long list of lesser problems.
There is a moose on the table, i.e. a fundamental dilemma that the authorities are apparently unable to resolve, or even to face squarely.
The books try to waltz around this fundamental dilemma. This produces all sorts of nonsense, including:
The problem is that all too commonly, the authorities give us rules that are grossly inconsistent with the examples they have given! Four such rules will be exhibited below.
This, alas, violates one of the most basic rules of scientific thinking. As discussed in reference 1, one should always consider all the data, or at least a fair sampling of the data. It is outrageous to base a rule on a handful of examples that have been selected or contrived to support the rule, while ignoring other examples that conflict with the rule. (See section 2 for examples relevant to the “chemical versus physical” discussion.)
The result is that students are being taught something that is a mockery of thinking and a travesty of science. They are learning a bunch of baloney that will have to be unlearned later. Any good teacher knows that unlearning something is an exceptionally difficult and unpleasant task.
Keep in mind that according to the authorities, the main point of the exercise is:
Fundamental Tenet: Naive students should be able, just by looking, to distinguish a “physical” change from a “chemical” change.These authorities say this distinction is manifested in examples ... and also in rules that unify previous observations and predict future observations.
Let us begin with a few conventional, widely-used examples. I indicate using the † symbol the ones that are partially or completely incompatible with a logical approach to the subject.
Example 1:
| Casually mixing iron powder with sulfur powder is a physical change. | Reacting iron with sulfur to produce iron sulfide is a chemical change. |
Example 2:
| Cutting a piece of paper with scissors is a physical change.† | Burning a piece of paper is a chemical change. |
Example 3:
| Opening a valve so that some compressed gas rushes out of its container is a physical change.† | Mixing acid with baking soda so that some gas rushes out of the container is a chemical change. |
Example 4:
| Cooling a mixture of air and kerosene vapor so that liquid kerosene condenses out is a physical change.† | Burning a mixture of air and kerosene vapor is a chemical change. |
Example 5:
| In general, any change of phase, such as liquid ↔ vapor, is a physical change.† | In general, any chemical reaction that converts one type molecular entity into another type of molecular entity is a chemical change. As a particularly simple example, consider the reaction 2F ↔ F2. |
Now let us see how far we can get if we attempt to form generalizations based on the available examples.
Attempted Rule A: Any change initiated by simple physical or mechanical means is a physical change.†
For instance, casual mixing is obviously a mechanical process. Similarly cutting with scissors is a mechanical process. Opening a valve is mechanical.
This rule runs into trouble as soon as we consider example 4. The physical change is controlled by a change in temperature. If you lower the temperature, the vapor condenses into liquid. If you raise the temperature, the liquid evaporates into vapor and mixes with the air. If you raise the temperature some more, the air/vapor mixture ignites – or explodes – which is a chemical reaction.
From this example, and many others, we learn that sometimes temperature initiates a physical change, and sometimes initiates a chemical change. This already calls Attempted Rule A into doubt.
Example 6:
Suppose we have an ordinary off-the-shelf bottle of carbonated water. If you remove the cap, gas rushes out. This seems closely analogous to example 3. But are we seeing a chemical change, or a physical change? It turns out we are seeing both. Before the bottle was opened, there was a four-way equilibrium:
- Some of the CO2 was in the gas phase, in the head space.
- Some of the CO2 was dissolved in the water, in the form of CO2. The dissolved CO2 was in equilibrium with the gaseous CO2.
- Some of the CO2 had reacted with the water, to form carbonic acid, i.e. H2CO3. The reaction CO2 + H2O ↔ H2CO3 was in nontrivial equilibrium, i.e. it did not go to completion in either direction.
- Some of the carbonic acid had ionized. The reaction H2CO3 ↔ H+ + HCO3− did not go to completion in either direction.
Now the point is that when you reduce the pressure by removing the cap, there are some obvious physical changes that take place, but there are also two chemical reactions that take place. At the new pressure, the chemicals are no longer in equilibrium, so some of the carbonic acid dissociates into water and CO2. Also some of the carbonate ions convert to un-ionized carbonic acid molecules.
Example 7:
This is the same as example 6, but rather than merely opening the cap and letting gas escape, we attach a piston to the top of the vessel. This allows us to raise and lower the CO2 pressure in the head space.When we do this, we discover that the system is reversible. A higher pressure of CO2 in the head space is associated with more CO2 in solution, and hence more H2CO3 in solution, and hence more H+ and HCO3− ions in solution.
At this point, it should be obvious that Attempted Rule A is deader than a doornail. Indeed, the Fundamental Tenet is also untenable. There is absolutely no way that an incoming student in an introductory-level course could, on the basis of casual macroscopic observations, tell whether example 6 depends on chemical changes, physical changes, or both. (Also, as mentioned in section 1, keep in mind that nobody cares.)
At this point, any sensible person would give up, since the Fundamental Tenet has been discredited. But chemistry texts rush in where angels fear to tread. Here’s another example:
Attempted Rule B: Chemical change involves breaking chemical bonds, whereas physical change does not break chemical bonds.†
This attempted rule cannot withstand scrutiny. It conflicts with example 2, since cutting a piece of paper consists almost entirely of breaking chemical bonds, i.e. cutting high-molecular-weight molecules into lower-molecular-weight molecules.
Some people try to salvage Attempted Rule B by claiming that the scissors (a) doesn’t cut any covalent bonds and therefore (b) doesn’t “really” cut molecules but rather cuts “between” molecules. First of all, part (a) of this claim is untrue, as you can see from example 8 and example 9 ... and even if part (a) were true, part (b) would violate the IUPAC definition of molecule. This salvage attempt cannot possibly succeed.
Example 8:
| Cutting a piece of nylon fabric with scissors is a physical change.† | Burning a piece of nylon is a chemical change. |
Example 9:
Suppose we start with a good-sized crystal of calcite. The crystal is beautifully clear. Split it using a geologist’s hammer. We now have have two crystals, just smaller. This process was carried out using an obviously “physical” means, but the most-important step in the process was the breaking of a huge number of chemical bonds in the crystal. If you keep pounding on the calcite with a hammer, you can quickly reduce it to a fine white powder.
There is no doubt that the nylon in example 8 consists of very long macromolecules – centimeters long, sometimes many meters long, covalently bonded from end to end – which can be readily cut by scissors. Similarly the macroscopic calcite crystal in example 9 is one huge covalently-bonded macromolecule. This can be cut into lower-molecular-weight molecules by purely mechanical means.
This leads us to the following:
Attempted Rule B2: Chemical change involves breaking many, many chemical bonds, whereas physical breaks relatively few (if any) chemical bonds.†
Minor point: This leads to unanswerable questions about how many broken bonds is “too many”. These questions are unanswerable because there will always be marginal cases, as you can see from example 9. There is no practical limit as to how finely the calcite crystal can be crushed, and therefore no practical limit as to how many bonds can be broken. This is an example of a “camel’s nose” argument. Once you allow a few bonds to be broken, there is no good place to draw the line.
Actually, the situation is even worse than that. There are two or three fundamental flaws in Attempted Rule B2. The first is a logical flaw: When you cut a piece of nylon with scissors, the bonds that are cut represent a small percentage of the total number of bonds ... but the bonds that are cut represent 100% of the cutting. So if we look at the cutting process per se, it consists entirely of chemical changes, even though it is routinely claimed to be “obviously” a physical change.
Another fundamental flaw is this: Not only is there no “good” way to decide how many broken bonds is too many, there is absolutely no place to draw the line that is consistent with the facts. That’s because there are processes that the authorities like to call “physical” where a majority of the chemical bonds are broken, as we see from the following examples:
Example 10:
Suppose we start out with a good-sized crystal of halite, which has empirical formula NaCl. The crystal is a single molecule, i.e. a macromolecule, ionically bonded. We can agree that the empirical formula is NaCl, and the unit cell formula is NaCl, but actual molecular formula is NaxClx, to a high degree of approximation, for some large value of x. Values on the order of x=1020 or even larger are commonly encountered.Now suppose we heat the crystal so that some vapor is formed. Under a wide range of conditions of temperature and pressure, the vapor will contain lots of NaCl molecules, that is, plain old Na1Cl1 molecules. This process is reversible, in the sense that if you lower the temperature, it is possible to regrow the crystal at the expense of the vapor.
In the NaCl molecules in vapor phase, each Na atom is bonded to exactly one Cl atom, and vice versa. In the crystal, each atom was bonded to its six nearest neighbors.
Example 11:
This is the same as example 10, but this time we use a covalently-bonded crystal such as silicon. The crystal is one big molecule. Each atom is covalently bonded to each of its four nearest neighbors. The vapor (under suitable conditions) consists of isolated silicon atoms – no bonds at all.
In example 10, for instance, you could say that 5/6ths of the ionic bonds are broken. In example 11, all of the covalent bonds are broken. These simple phase changes are widely considered to be physical changes, yet there is no way they can be construed as breaking only a “few” of the bonds.
You could try to defend the idea of chemical versus physical change by saying that any bonds that get broken when something evaporates will – arbitrarily – not be counted. However, even that last-ditch defense leads to logical inconsistencies, as we see from the following example, which is closely related to example 5:
Example 12:
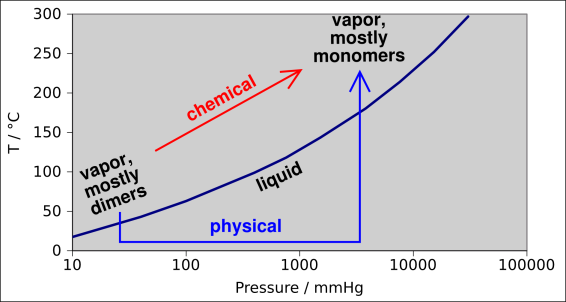
| The liquid ↔ vapor phase change in anhydrous acetic acid is a physical change.† | A chemical reaction such as 2 CH3COOH ↔ (CH3COOH)2 is a chemical change. |
We now invoke the principle that a process that consists of two or three physical changes is itself a physical change. This principle often goes without saying, but it remains true and important. This is called the limited transitive property.
Note that I am not claiming an “unlimited” transitive property. We know that a process that breaks a few bonds, if repeated enough times, will break a great many bonds. This is the minor point made above, and is not the point that I wish to emphasize.
A more interesting and more fundamental argument can be made as follows: It turns out that in the vapor phase, acetic acid tends to form dimers, i.e. (CH3COOH)2 ... especially at low temperature and/or low molar volume. This is analogous to the dimerization reaction mentioned in example 5, but perhaps more familiar, because it occurs under more convenient conditions of pressure and temperature.
Now if you believe that a liquid ↔ vapor phase change is a physical change, we have a clear violation of transitivity. We have two clear-cut examples of supposedly physical changes that, in combination, are equivalent to one clear-cut chemical change. The situation is diagrammed in figure 1.
The fundamental problem here is that when people classify phase changes as physical changes, they are implicitly assuming that the forces that hold crystals together – and the forces hold liquids together – are somehow different from the forces that hold molecules together. They are assuming something that is fundamentally not true.
Believe it or not, even after all that, there are people who still try to defend Attempted Rule B. They say it is important, because of the following:
Attempted Supporting Rule: molecules are the “stable particles of matter”† and therefore deserve special consideration.
Alas, that attempted supporting rule is a non-starter, as illustrated by example 13.
Example 13:
Suppose we have a container of ordinary water sitting on the shelf. We just let it sit there. Conditions of temperature, pressure, etc. do not change. You might think that by any reasonable definition, no physical change is occurring, and no chemical change either.However, note that at standard temperature and pressure, about 18 ppb of the water is auto-ionized, i.e. dissociated into H+ and OH− ions. (The ions are, of course, solvated.) Ions are recombining and new ions are being formed on a very rapid timescale.
If you add heavy water (D2O) to ordinary water, you will very quickly wind up with a lot of HDO molecules.
Water molecules are not “stable particles of matter”. Let’s be clear about this:
So let’s give up on Attempted Rule B.
At this point, you may be convinced that trying to distinguish physics from chemistry is a pointless exercise. If so, you can stop reading now. However, experience shows that some people are not yet convinced.
Attempted Rule C: A chemical process (unlike a physical process) produces a product with different properties than either ingredient.†
This rule is, alas, quite unreliable. There are many counterexamples, including example 9. The powdered calcite has several properties not shared by the original large crystal. For starters, it is a different color. Other counterexamples include example 14, example 15, and example 16.
Example 14:
Suppose we take some copper and alloy it with a little bit of tin. Most people consider this a purely physical mixture. That is to say, there is no sign of any intermetallic compound being formed. However, the product – called bronze – has properties are definitely not what you would predict just by averaging the ingredients:
- It has a lower melting point than either ingredient.
- It has a different color.
- It has different thermal-expansion properties.
- It has greater hardness than either ingredient.
- It has wildly different electrical conductivity, especially at low temperatures.
Example 15:
As a more prosaic example, consider what happens if you mix the yellow dye from an ink-jet printer with the cyan dye. The mixing process is not a chemical process; basically it is just a mixture, rather like example 1.However, the mixture has a different color than either ingredient: it’s green.
Example 16:
Perhaps an even better example is the following: Start by asking students what what was the color of Napoleon’s white horse. This is a silly question ... a proverbially silly question. The answer, of course, is white. Next, ask them what color is the stuff that comes in an ordinary bottle of yellow food coloring. Many of them will assume that it is yellow. A few of them may know, based on experience, that the right answer is otherwise. In fact, the stuff in the bottle is red. Really, deeply red. If you dilute it enough, it becomes orange, and if you dilute it even more, it becomes yellow.If you do the experiment, many students will tell you that diluting the yellow food coloring must involve some sort of “chemical change” because they’ve been taught that color change implies chemical change. That is, alas, dead wrong in this case.
To understand what is really going on, dilute the yellow food coloring to a moderate degree and put it in a white bowl with sloping sides. You will observe that in thin layers, the liquid is perceived as yellow, while in moderately thick layers it is perceived as orange, and in yet thicker layers it is perceived as red.
Remark #1: It should be obvious that there is no “chemical change” involved in going from a thin layer to a thick layer.
Remark #2: There are good reasons – excellent physical reasons – why the thin layer should have a different perceived color from the thick layer. The physics of light absorption is nonlinear. See reference 2.
Saving the strangest for last, we have this gem. Some people advocate the following rule:
Attempted Rule D: Chemical changes are irreversible, while physical changes are reversible.†
(I’m not making this up, some people really advocate that!)
This rule is supported by example 1, but contradicted by a host of other examples. For example, the chemical reaction mentioned in example 5, namely 2F ↔ F2, is commonly carried out under conditions where it is reversible for all practical purposes. Raising the temperature and/or raising the molar volume shifts the equilibrium to the left, whereas lowering the temperature and/or lowering the molar volume shifts the equilibrium to the right. Note that this provides another disproof of Attempted Rule A, since we have a chemical reaction that can be initiated – in either direction – by a simple, physical, mechanical change, i.e. changing the volume.
It may be that the first few reactions that you saw in high-school chemistry class were irreversible, but in the real world, many reactions proceed both forwards and backwards. Example 7 is is another illustration; example 17 is yet another.
Example 17:
Consider the electrochemical reactions in a storage battery. By the physical process of turning the crank on a dynamo, you can run the reactions forward. By turning the crank the other way, you can run the reactions backward.
The other half of Attempted Rule D is wrong, too; there are plenty of so-called “physical” processes that are, in practice, irreversible. Crushing a calcite crystal to powder (example 9) is irreversible; there is no easy way to un-crush it. Similarly, cutting paper with scissors (example 2) is irreversible; there is no way to use the scissors “backwards” so as to un-cut the paper. More importantly, there are plenty of truly physical processes, involving no chemical changes whatsoever, that are irreversible. Stirring a liquid is thermodynamically irreversible. An ideal gas rushing through an orifice from a high-pressure region to a low-pressure region is thermodynamically irreversible. Heat flow along a bar that is hot at one end and cold at the other end is thermodynamically irreversible. Other examples abound.
The preceding discussion should make it clear that there is no useful distinction between chemical change and physical change ... except possibly in a few extreme cases.
Any students who are dissatisfied with the usual “textbook” discussion of this topic should be congratulated. It shows they have some critical-thinking ability.
Too much of intro-level chemistry is devoted to rote learning of notions that are not really correct, and would be worthless even if they were correct.
We should concentrate on meaningful rather than meaningless classifications.
Let me explain the meaning of this metaphor:
| There is a sharp, legal line between Kansas and Colorado. They do not overlap. The line is, however, arbitrary. It has no effect on the geological “ground truth”. | There is tremendous overlap between “physics” and “chemistry”. There is no sharp dividing line. Even if somebody managed to lay down such a line, it would be completely arbitrary. It would have no effect on the ground truth. The atoms and molecules are what they are, and they do what they do. They do not recognize any distinction between chemistry and physics. |
In some jurisdictions, teachers are required to cover the topic “chemical versus physical change”. Sometimes this requirement is direct, and sometimes it is indirect, in the sense that the topic is included on the mandatory standardized tests.
I don’t recommend ducking the issue. We need to teach students how to deal with bad ideas that are likely to show up on the test. I take the direct approach. I tell them:
- If it’s not worth doing, it’s not worth doing right.
- Don’t pollute your brain.
Here’s what I mean by that: Ordinarily, the right way to learn things is to take each new idea and turn it over in your mind, to see how the new idea is connected to everything else you know. However, when faced with a pseudo-idea that doesn’t make sense, you should not try to make sense of it. Just learn the pseudo-idea by rote. Don’t think about it. If you think about it, you will just pollute your brain.
Also: ordinarily cramming is a bad idea. Anything you “learn” one hour before the test you will forget one hour after the test. However, for pseudo-ideas, that’s just what you want. There are only a few pseudo-ideas that show up on the test, and I can tell you what they are, so make a list. Cram them into rote memory right before the test, and forget them right afterward. The test cannot possibly ask any deep questions about these pseudo-ideas, so rote memory will be quite sufficient.
Meanwhile we should agitate to get the nonsense removed from the tests.
Also: Remember that elections have consequences. Find out about the candidates, even in off-year elections, and even in down-ballot races such as school board. Then be sure to vote. Otherwise the wackos will get elected.
Please let’s stop foisting ideas of physical versus chemical change onto the students. Those ideas are worse than worthless. The less said about them, the better.
The only reason why anyone would reasonably be interested in such ideas would be if they lived in the 19th century, before there was any useful understanding of atoms.
The 19th century has been over for a while now. Wake up and smell the atoms. Anything you could possibly explain in terms of a physics-versus-chemistry distinction can be explained infinitely more clearly by saying what the atoms and molecules are doing. Especially in an introductory course, students should see the best evidence, not the most ancient evidence. See reference 3. A useful valid microscopic story is preferable to a useless invalid macroscopic story.
There is a saying, “You can’t beat something with nothing”. The question therefore arises, if we aren’t going to spend the first week of class talking about “chemical” changes versus “physical” changes, what should we talk about instead?
The answer is simple: Talk about real things. Talk about protons, neutrons, and electrons, and how they form atoms (reference 4). Talk about atoms and bonds and molecules. See reference 5 for suggestions on how to introduce such ideas at the high-school or even pre-high-school level. Talk about energy and entropy (reference 6). Talk about dimensional analysis and scaling laws (reference 7 and reference 8). Talk about things that really matter.