Our definition applies easily and correctly to every situation I can
think of (with one execrable exception, as discussed item 11
below).
Crucially, our definition applies just fine to things like a
rechargeable battery, where you cannot identify the anode and cathode
until you see how the device is being operated, as discussed in
item 6.
Our definition also applies just fine in cases where it is relatively
easy to distinguish anode from cathode “just by looking” as
discussed in item 7.
-
Ours is the original, time-honored definition. It
is consistent with the etymology, as discussed in item 17.
There is no other sensible definition. I’ve seen several attempts at
definitions, but unless they were equivalent to our definition (as
given in section~1), they were grotesquely overcomplicated,
wrong, or both.
-
By well-established convention (going back to
Ben Franklin), when we speak of current we mean the conventional
positive current. In metal wires, the current is carried by
electrons moving in the direction opposite to the current. This
complicates the notion of current, but is necessary because the
electron is negatively charged.
-
For the vast majority of people, there is no
point in memorizing the meaning of anode and cathode. The terms just
aren’t very useful, unless you get a job in an electrochemistry
laboratory or some comparably narrow specialty. If some day you do
need to know the meanings, you can look them up that morning and
forget them again that evening.
-
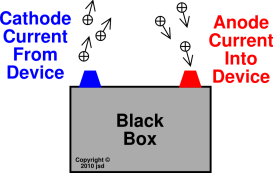
Note that when we say current-in, we mean current
flowing into the device from the external circuit. Similarly when we
say current-out, we mean current flowing out of the device toward the
external circuit. We are treating the device as a black box, and we
are emphatically not talking about whatever currents flow within the
device. This black-box terminology is standard in all branches of
engineering and science, unless the context clearly requires
otherwise.
If you insist on peeking inside the black box, the story gets more
complicated, as you can see in figure~2. However,
this does not change the letter or the spirit of the definition, which
is based on the behavior of the black box, as seen from the outside.
-
It is crucial to remember that the anode/cathode
distinction is based on current, not voltage. Anode/cathode is not
the same as positive/negative or vice versa. Illustrative example:
for a battery being discharged, the positive terminal is the cathode,
while for the same battery being recharged, the positive terminal is
the anode.
-
As a trustworthy rule, keep in mind that
anode and cathode refer to function, not structure. There are lots of
devices where it would be madness to permanently label the structures
as anode or cathode, because their function changes from time to time.
Rechargeable batteries are a common, very important example,
as mentioned in item 5.
-
Although anode and cathode are
fundamentally defined in terms of function not structure, there
are some exceptional devices where the function is essentially locked
to the structure. In such a case, it is arguably permissible to label
the structures as anode and cathode, because only one direction
of current-flow makes sense. On the list in section~2, all the
examples except for the rechargeable battery are in this
category.
In any case, keep in mind that this category must be considered the
risky exception, not the general rule. The trusty general rule is
explained in item 6.
-
Even in cases where it is arguably
possible to identify a definite anode and cathode, there are usually
simpler and better ways to designate the terminals. Specifically, for
a battery (rechargeable or not), it is conventional and sensible to
speak of the positive terminal and negative terminal. For a diode, it
is conventional and sensible to speak of the P-doped side and the
N-doped side. In particular, for a LED display module, the so-called
common-anode configuration would more properly be called the
common-P-side configuration.
-
Here’s an interesting and important example: Consider
the electrolytic refining of metals such as copper.
During normal operation, a large current flows through the cell,
imposed from the outside. Current is pushed into the cell at the
anode, and taken out at the cathode. The terminals are labeled
according to their normal function, in accordance with the definition
given in section~1.
At the beginning of the operation, the anode is impure copper. At the
end of the operation, the cathode is much higher purity copper. Try
googling for anode
mud.
If some wise guy temporarily reversed the direction of the current,
the normal anode would become the temporary cathode and vice versa.
However, this possibility is so kooky that it is usually not even
considered. The terminals are labeled according to their normal
function.
Note the contrast:
|
Electrolytic refining cell.
|
|
Ordinary battery
|
|
In the refining cell, the open-circuit cell voltage, if any,
is very small and completely irrelevant.
|
|
In the battery, there is a
definite positive terminal and a definite negative terminal.
|
|
The voltage drop across the cell is roughly proportional to
the current. During operation, the anode will be at a positive
voltage relative to the cathode.
|
|
The voltage drop across the cell is
qualitatively the same, no matter whether the current is positive,
negative, or zero. The positive terminal is the cathode during
discharge, but it is the anode during recharge.
|
-
In all cases, you can use the descriptive terms
current-sink and current-source as synonyms for anode and cathode.
Description is generally preferable to jargon.
-
It is possible to buy an array of Zener diodes.
Alas, according to an established but illogical convention, the
so-called common-anode configuration is structurally analogous to a
common-anode array of LEDs, in the sense that the P-doped sides are
tied together. This is an abomination, because in normal Zener usage,
the P-doped side is where the current exits, and should logically be
called the cathode. Apparently somebody was under the misimpression
that anode/cathode referred to structure instead of function.
You should never use the terms anode or cathode to describe the
structural parts of a Zener diode, for the same reason you should not
use such terms for the structure of a rechargeable battery. Anode and
cathode refer to function, not structure. Instead you should refer to
the P-doped side and the N-doped side, and you should insist that
others do the same.
Note that reversing the labeling convention for Zener diode arrays
would not solve the problem in any fundamental sense, because there
are perfectly reasonable circuits in which – part of the time – a
Zener diode is forward biased, so that it conducts just like any
other diode. This is the same situation we encounter in connection
with rechargeable batteries: if you attach permanent anode/cathode
labels to the structure, you will be wrong at least part of the time.
The terms “anode” and “cathode”
properly apply to function, not structure.
|
|
| ~~~~~
|
-
Electrochemical corollary: In any electrochemical
cell, oxidation reactions take place at the anode, and reduction
reactions take place at the cathode. (If you don’t know what this
means, don’t worry about it.) This includes batteries being charged
(anode=positive) as well as batteries being discharged
(anode=negative). This is a corollary flowing from our definition,
and from the conventional viewpoint that the cell is the black box,
and everything external to the cell is the external circuit.
The situation is summarized in the following table:
|
~ | ~~~~~ | charging | ~~~~~ | discharging |
| ~~~ | ~~~~~ |
| − plate: | ~~~~~ | cathode
being reduced | ~~~~~ | anode
being oxidized |
| ~~~ | ~~~~~ |
| + plate: | ~~~~~ | anode
being oxidized | ~~~~~ | cathode
being reduced |
-
Let us make a brief exception to the black-box
viewpoint, and consider what happens inside an electrochemical cell.
Inside the cell, cations (positively charged species) moving toward
the cathode make a positive contribution to the conventional current
inside the cell, as shown in figure~3.
Similarly, anions (negatively charged species) moving toward the anode
make a positive contribution to the conventional current inside
the cell. No anions are shown in the figure. The rule
anions-to-anode, cations-to-cathode applies only inside the cell.
This rule is required by the fact that current obeys a conservation
law; current that flows into the cell at the anode must flow through
the cell and then out the cathode. Outside the cell, current flows
toward the anode; inside the cell, current flows away from the anode.
(By the way, it is usually assumed that outside the cell, there are no
mobile anions or cations, just electrons carried by metal wires in the
external circuit.)
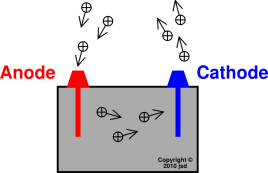 Figure~3
Figure~3: Anode and Cathode : Inside the
Black Box
When talking about ions, you need to remember that cations are
positively charged. The mnemonic for cations is to view the ‘t’ as a
plus sign: ca+ion. Meanwhile, the mnemonic for anions is something of an
acronym: A Negative ION = ANION.
When remembering the cations-to-cathode rule, you need to remember
that inside the cell, cations go to (not from) the cathode: ca+ions
+o ca+hode. The corresponding anions-to-anode rule is equally
valid, but you have to work harder to remember that the anions go to
(not from) the anode.
Please remember that the cations-to-cathode rule is subject to
multiple caveats. It is at best a chemical corollary to the real
definition. It cannot possibly serve as the definition of cathode,
because the cathode is well-defined for all sorts of devices that
don’t have any mobile cations, e.g. semiconductor diodes, cathode-ray
tubes, et cetera. Another caveat is that this rule applies to what’s
going on inside the cell, whereas for most purposes (including the
anode/cathode definition) it is conventional and appropriate to focus
on the properties of the black box, as seen from the outside. (Similar
issues arise in item 14 and item 16.)
-
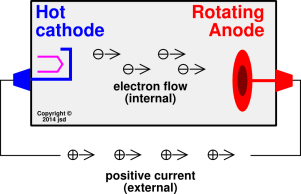
There is some slight potential for confusion when
thinking about cathode ray tubes and X-ray tubes, because of the
temptation to deviate from the black-box viewpoint. (Similar issues
arise in item 13 and item 16.) In an
X-ray tube, the interesting physics is happening inside the device,
whereas the definition of anode is expressed in terms of conventional
current flowing into the external terminal, flowing into the black box
from outside. Remember, from outside the device we see positive
conventional current coming out of the cathode and going into the
anode, in accordance with our definition, as seen in figure~1 in section~1. The rule is ACID: Anode
Current Into Device. (If we peek inside the device, we see electrons
streaming out of the cathode, but that’s only a corollary of the
definition, not the definition per se.)
-
There is even more potential for confusion if
you try to explain or define anode/cathode in terms of electrochemical
cells, if only because very very few people understand how such things
work. See reference~1 and references therein. As the saying
goes, learning proceeds from the known to the unknown. Our definition
of anode/cathode, as given in section~1, is simple and useful.
The internal mechanism of a battery is not simple. It makes no sense
to “explain” the former in terms of the latter.
Battery terminals are labeled positive and negative. They are labeled
according to voltage, not charge or current. This is conventional and
entirely appropriate, because the positive terminal remains at a
positive voltage (relative to the other terminal) during all normal
conditions, including when the battery is discharging, recharging, or
just sitting there in equilibrium with no current flowing.
In contrast, as mentioned in item 5, it would be wildly
inappropriate to label the battery terminals as anode and cathode.
That’s because the terminal that is the cathode during discharge
becomes the anode during recharge ... and is neither anode nor cathode
in the equilibrium (no current) situation.
Furthermore, it makes no sense to define anode and cathode in terms of
electrochemisty, because the terms are used in all sorts of situations
where there is no electrochemistry involved, including semiconductor
diodes, X-ray tubes, et cetera.
-
Boats and other structures in contact with
salt water give rise to a situation with some potential for confusion
about anode versus cathode. At first glance it might not be obvious
what’s considered the “black box” and what’s considered the
“external circuit”. The conventional viewpoint is this:
- The water, and the metals touching the water, are to be thought
of as a giant electrochemical cell. There are anions and cations in
the water, inside the black box.
- The structure of the boat (or whatever) is considered the
external circuit. There are no anions or cations. The current is
carried by electrons flowing inside metals.
That is to say, the convention is to consider the boat as external to
the water ... even though it might seem more logical to think of the
water as external to the boat. This may seem arbitrary, but at least
it is consistent with the aforementioned electrochemical corollary
(item 12), so that oxidation reactions take place at the anode,
and reduction reactions take place at the cathode. This leads us to
the useful concept of a sacrificial anode, which is just a
cheap, easily-replaceable electrode that is placed in the water and
arranged to have a large positive voltage with respect to the rest of
the boat. That makes everything else on the boat a cathode, greatly
reducing corrosion, because most forms of corrosion involve oxidation
reactions. To say the same thing in other words, inside the water,
highly corrosive anions such as OH– and Cl– are flowing toward
the anode and away from everything else, in accordance with the
anions-to-anode rule. The anode, of course, corrodes rapidly, and
needs to be replaced on occasion.
-
Etymology: The words anode and cathode were
introduced in 1834 by Michael Faraday on the advice of William
Whewell, the polymathic scientist and prolific wordsmith. Whewell
understood quite a bit of Greek, and put it to good use:
Anode comes from the Greek roots ἀνά +
ὀδός (meaning upward path).- Cathode comes from the Greek roots κατά
+ ὀδός (meaning downward path).
One should never place too much emphasis on etymology, because
meanings can drift over time. Indeed ἀνά and
κατά have drifted from their ancient roots.
However, ὀδός has not, and
that’s the key. The English words, when coined, were clearly intended
to describe flow, not voltage. The same roots are used in other Greek
and pseudo-Greek terms in English, e.g. anabolic, cataract, odometer,
et cetera.
I am astonished that some people take a concept that is simple and
unimportant, make it needlessly complex, and pretend it is important.
When dealing with batteries, don’t think in terms of anode and
cathode; think in terms of positive terminal and negative terminal.
When dealing with semiconductor diodes, don’t worry about anode and
cathode; think in terms of P-doped side and N-doped side.
There is abundant evidence that even people who call themselves
experts cannot keep the anode/cathode terminology straight. In any
practical situation, there is always a way to figure out how to hook
things up without a deep understanding of anode versus cathode.
The terms anode and cathode are sometimes convenient, in situations
where only one direction of current makes sense.
In other situations, it is usually better to avoid the terms anode and
cathode. There are better ways to say what needs to be said.
Constructive suggestion: it is better to talk about the current
(rather than the electrode). It is better to talk about what the
current is doing (rather than what the electrode “is”).