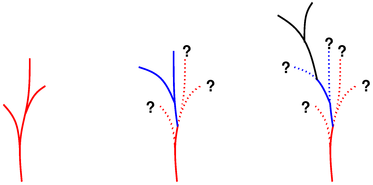
Figure 1: Back-Tracking
Abstract
As a rule, any introductory course should keep things simple and straightforward. Exceptions to this rule should be rare.
Alas, real history is neither simple nor straightforward. As discussed in section 3, the true history of science is a tale of confusion, with much backtracking out of blind alleys. As discussed in section 2, we should study real history ... not fake history.
The surprising thing is that many teachers claim to use “the historical approach” to organize and motivate the study of science. As discussed in section 1, this simply cannot be an accurate description of what they are doing, since nobody would be so foolish as to inflict the real history on introductory-level students.
There is a moose on the table, i.e. a huge stinky problem that some people would like to ignore, but really cannot be ignored. Actually it is a group of related problems:
The aforementioned technical problems lead directly to a series of pedagogical problems, when you are deciding how to present the material:
As pointed out by Kuhn (reference 1) and others, cherry-picking the correct speculations is all too common. The “history” taught in introductory science class bears little resemblance to the actual historical facts. In many cases, folks who advocate the “historical approach” are so ignorant of the real history that they don’t realize how badly they are twisting the facts.
This is not a feasible way to achieve historical accuracy, because there simply isn’t enough time in the day to pursue a historically-accurate sample of the erroneous and/or low-value ideas.
If you try this, the most likely outcome is a lose/lose proposition: Enough historical detail to seriously detract from the proper goals of the course, but not enough to give an accurate portrayal of the history.
Every time you suspend critical judgment in order to follow the historical footsteps, you are just reinforcing a bad habit, namely the habit of not thinking. As with most unsound decisions (such as driving without a seat belt), the fact that you sometimes get away with it doesn’t make it a sound decision.
It’s easy for experts to overvalue the historical literature. That’s because they know how to pick out the good bits and ignore the rest. In contrast, the students in an introductory course cannot possibly know which bits they should absorb and which bits they should ignore.
There is no law that says pedagogy must recapitulate phylogeny. In introductory classes we should use the best available evidence, not the most ancient evidence.
I have the greatest respect for real history, real historians, and real historical figures. If someone really wants to study the history of science, that’s commendable. The history of science is an advanced topic, suitable for those who already have a good grasp of science and a good grasp of historical methods. Please do not confuse introductory physics with the history of physics.
We should show our respect for real history by rejecting bogus history.
The proper goals of history are primarily to say what happened, and secondarily to explain what happened. History does not consist merely of arranging some things in chronological order. In particular, the history of science is not a chain of successively more-refined ideas arranged in chronological order; it is more like the plant in figure 1, including all the dead ends. The dead ends are more than half of what really happened.
When writing history, real historians hold themselves accountable for what they leave out, not just what they put in. A story that ignores the dead ends is not history.
Changing the name from “the historical approach” to “the chronological approach” would be a small step in the right direction, but would not solve the whole problem, or even the main problem. Students were not born knowing the distinction between chronology and history, so you would have to explain to them in some detail why the chronological approach they see in the textbook is an extreme distortion of history. And even then, some of them will not appreciate the distinction. Furthermore there would remain pedagogical problems, because usually the logical progression of ideas differs greatly from the chronological progression of ideas (let alone the true historical progression).
Physics teachers do not have a license to falsify the history, any more than history teachers have a license to falsify the physics.
Science is like a plant that grows some during the summer but suffers quite a lot of die-back during winter. The next summer, it grows again, sprouting not from the tips of last year’s growth, but from somewhere much farther back along the stem.
This is illustrated in figure 1. The left diagram shows the situation after the first summer: The first year’s growth is shown in red.
The middle diagram shows the situation after the second summer. Much of the red growth was killed during the winter. The second-year’s growth is shown in blue. We emphasize that this growth is not an extension from the tips of the red growth; it sprouts from somewhere much farther back along the stem.
The right diagram shows the situation after the third summer. Much of the blue growth was killed during the winter. The third year’s growth is shown in black. It is not an extension from the tips of the blue growth; it sprouts from somewhere well away from any of the previous tips. Furthermore, it does not come from what originally appeared to be the main stem, but rather from one of the side-branches.
This metaphor applies to the history of science as follows: If you just trace the direct path from the root to the current growing tip, you are bypassing more than half of the true history. The result is distorted and deceptive.
A great deal of research involves exploring blind alleys and then backtracking out of them. This is inefficient, but necessary, because we don’t know the alley is blind until we have explored it. We wish we could do things more efficiently, but wishing does not make it so.
“The progress of science is much more muddled than is depicted in most history books. This is especially true of theoretical physics, partly because history is written by the victorious. Consequently, historians of science often ignore the many alternate paths that people wandered down, the many false clues they followed, the many misconceptions they had. These alternate points of view are less clearly developed than the final theories, harder to understand and easier to forget, especially as these are viewed years later, when it all really does make sense. Thus reading history one rarely gets the feeling of the true nature of scientific development, in which the element of farce is as great as the element of triumph.”– David J. Gross
Nobel Lecture (2004)
opening words
In the study of history, the so-called “great man theory” is based on the assumption that the main features of history are due to the actions of a few great men: kings, military heroes, a few famous scientists, et cetera. This theory was controversial even in its heyday, about 100 years ago, and is completely discredited today. See reference 2.
However, people who have no understanding of history keep re-inventing this idea. It is a monumentally bad idea, for a number of reasons.
For one thing, it is a form of appeal to authority, which is almost always a bad idea, as discussed in reference 3.
For example, Newton made epochal contributions to mathematics and physics. That makes him a Great Man. Does that mean we should believe everything he had to say about alchemy? I don’t think so. Yet variants of this argument persist. I’ve heard professors argue with a straight face that Mendel was a Great Man who made epochal contributions to genetics, and therefore his data “couldn’t” have been fudged to better fit simple theory.
Another manifestation of this idea is that people who ought to know better commonly attribute to Newton all sorts of ideas that were figured out by Galileo and others. Similarly, they attribute to Einstein all sorts of ideas that were figured out by Galileo, Lorentz, Poincaré, Minkowski, Debye, and others.
The notion that such-and-such piece of physics could be the work of just one person is almost always false. It makes the job of doing science seem simultaneously easier and more magical than it really is. This is an insult to all scientists, past and present. It is also a disservice to students who might be thinking of going into science, because it gives them a false impression of what the job is like.
Furthermore, assigning all the credit to the most well-known contributor interferes with scientific teamwork. No less-famous person is going to collaborate with a more-famous person, if the more-famous person is going to get all the credit (whether he wants to or not). This is harmful to the careers of both people involved, by limiting their ability to collaborate. It also makes trouble for the research manager who is trying to set up collaborations.
Here’s a constructive suggestion: Given the irreconcilable conflict between the logical development and the historical development of the subject, one option is to do both – which is OK, provided you do them separately, and respect the limitations.
That is, if you want to diagram the logical relationships between the ideas, you should draw a concept map, without reference to history. Later you can also show the timeline if you wish – just do it separately, and make sure everybody understands that the timeline is chronology, not history.
We now turn to some specific examples of good and bad ways of doing things.
The right way: We can ascertain the existence of atoms – and the size of atoms – by looking at them with a good scanning tunneling microscope (“STM”), as shown in figure 2.
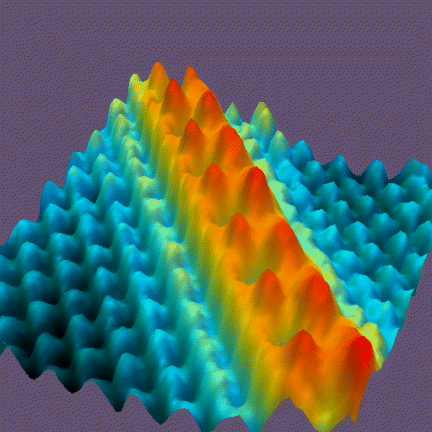
I don’t want to get into a metaphysical debate over whether the bumps seen in figure 2 “are” atoms. It suffices to say that the bumps are in one-to-one correspondence with atoms, and that the spacing between bumps tells us the spacing between atoms.
For more about the modern evidence for the existence of atoms, and for the size of atoms, see reference 5. For excellent information on how to apply modern atomic thinking at the high-school or even pre-high-school level, see reference 6.
The wrong way: The true history of the “atomic hypothesis” goes back thousands of years, but consists mostly of ill-founded speculation.
The first guy to quantify the size of atoms was Loschmidt. Although the raw data he used came from experiments that are not unduly complicated, the problem is that interpreting the data in terms of atoms requires a degree of theoretical sophistication that is grossly mismatched to an introductory course at the high school or college level. Therefore relying on this historical evidence (rather than the modern AFM evidence) is not the recommended approach.
Similarly, Einstein showed that the color of the blue sky provides an estimate of the size of atoms. Again, anyone can observe the raw data, but interpreting the data in terms of atoms requires a degree of theoretical sophistication that is grossly mismatched to an introductory course. Again: relying on this historical evidence (rather than the modern AFM evidence) is not the recommended approach.
Message: Especially at the introductory level, students should see the best available evidence, not the most ancient evidence.
The Right Way: The simplest and most useful way to understand special relativity is to see it as the geometry and trigonometry of spacetime – nothing more and nothing less. This can be quantified in terms of spacetime diagrams, proper time, proper length, invariant mass, and four-vectors. See reference 7 for details and further references.
This approach has the side-benefit of reinforcing students’ understanding of ordinary Euclidean geometry, trigonometry, symmetry, and three-vectors.
There are no “relativity paradoxes” when you do things the right way. Paradoxes arise only when the laws of physics are mis-stated. The true laws of physics are (so far as we know) paradox-free.
The Wrong Way: Those who adhere to the “historical approach” are obliged to introduce the idea of FitzGerald-Lorentz contraction. This is an idea that predates Einstein’s 1905 special-relativity papers by several decades.
This leads to a great deal of tortured logic and tortured terminology. It involves rulers that can’t be trusted, clocks that can’t be trusted, and multiple inconsistent notions of velocity-dependent mass. It leads to paradoxes galore.
This whole mess is utterly unnecessary. It has been unnecessary for 100 years, ever since Minkowsky’s magnificent spacetime paper (reference 8).
Some people think thermodynamics is all about heat, but it’s not. If you want a modern understanding of thermodynamics, the less fussing with heat, the better.
By way of analogy: As discussed in reference 1, phlogiston was a good idea in its day, but its day has come and gone. It has long-since been replaced by two ideas, namely oxygen and energy.
So it is with heat. It was a good idea in its day, but its day has come and gone. It has long-since been replaced by two ideas, namely energy and entropy. Time spent trying to quantify heat is worse than useless. Anybody who is serious about solving real-world problems will quantify the energy and entropy.
The right way: Starting in grade school, introduce ideas of probability. Play games and do quantitative experiments that involve dice-rolling and coin-tossing.
Then entropy can be defined in terms of probability.
Then thermodynamics can be discussed in terms of energy and entropy. For details, see reference 9.
The wrong way: The true history of science includes a long, long episode where people thought heat was conserved. Students who are led along the historical path will see a mountain of historical evidence that heat is conserved. They will be able to connect this with evidence from the own lives, coming from situations where heat is approximately conserved. This is a problem, because heat is not conserved in general. At some point, students will have to unlearn most of what they have learned about heat. Unlearning is always difficult.
Do you really want to follow the historical approach, or even the chronological approach? Are you sure?
If anybody really believed that, they would present the Maxwell equations (1864) before they introduced the idea of vector cross product (1880s). But I doubt anybody is crazy enough to do that. Maxwell’s original paper (reference 10) is very difficult to read, because it doesn’t use vector ideas.
What’s wrong with this picture?
History proves it must be possible to use Green functions without using delta functions, but it would be insane to do so today, especially in an introductory course.