1 Pressure in Neutron Stars, Metals, Atoms, etc.
The big questions for today are:
-
Why do neutron stars exist?
- Why is the speed of sound much higher in
metals than in ideal gasses?
- Why do atoms not collapse and become
100,000 times smaller than they are?
In discussions of such issues, one hears a lot
about “degeneracy pressure”. Similarly, in chemistry and
condensed-matter physics, one hears a lot about the “exchange
interaction”.
So, to make progress on the big questions, we need to understand:
- A) What is this so-called “degeneracy” pressure, and
- B) Should the exchange interaction
be considered “fundamental” – on the
same footing as the gravitational interaction, the strong nuclear
interaction, et cetera?
Executive summary: There is nothing special about “degeneracy” pressure.
It’s just pressure.
-
A non-degenerate gas has pressure. It is compressible.
- A partially-degenerate gas has pressure. It is compressible.
- A degenerate gas has pressure. It is compressible.
The only halfway-special thing is that when there is degeneracy, the
pressure will be different from what a naïve classical model would
have predicted. For a Fermi gas, it will be higher. In particular,
the pressure of a degenerate Fermi gas doesn’t go to zero at low
temperatures.
This document is also available in
PDF format. You may find this advantageous if
your browser has trouble displaying standard HTML math symbols.
As the saying goes, learning proceeds from the known to the unknown.
We can’t attack the aforementioned big questions until we have a clear
idea of what degeneracy is, what pressure is, and why a fundamental
force is called fundamental. Here are some smaller questions and
answers that may serve as useful stepping-stones in preparation for
answering the big questions.
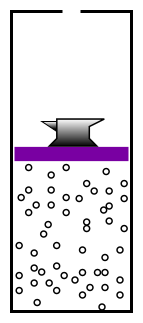
- Question 0: In figure 1, why doesn’t the anvil
descend all the way to the bottom? It would have lower gravitational
potential energy there. The system should go to a low-energy state.
Answer: If the anvil went down, the microscopic kinetic energy of the
gas molecules would go up. We have a name for this change in gas
energy as a function of volume: It’s called pressure.
- Question 1: Is this fundamental? Why is kinetic energy not included
on the usual lists of “fundamental interactions”?
Answer: Kinetic energy is always part of the equations of
motion. It is not listed as a fundamental interaction, because it
is not an interaction. It is present even in the absence of
interactions, including when there is only one particle, or when
the particles are so far apart that they don’t appreciably
interact. In some sense it is more fundamental than the
so-called “fundamental interactions”.
See also item 12 below.
- Question 2a: How big is a proton, a hydrogen nucleus?
Answer: about one Fermi, 10−15 meters.
- Question 2b: How big is an electron?
Answer: zero. For purposes of atomic physics, an
electron is a point particle. No structure.
- Question 2c: How big is a hydrogen atom?
Answer: Roughly one Ångstrom, 10−10 meters in diameter.
The atom is fluffy and squishy, so it’s hard to measure (or even
define) “the” exact diameter, but this is a reasonable estimate.
- Question 2d: What’s the electrostatic potential energy for an electron when it
is about half an Ångstrom away from a proton?
Answer: About −29 eV (measured relative to a widely-separated
electron and proton). You can calculate this directly from
Coulomb’s law.
- Question 2e: What’s the actual binding energy for the electron in a
ground-state hydrogen atom?
Answer: One rydberg, 13.6 eV. This can be measured
super-accurately by spectroscopy. It can also be calculated from
first principles, if you know enough about atomic structure. This
number is markedly less than the electrostatic energy mentioned in
item (2d), because we have to take the kinetic energy into
account. There’s a lot of kinetic energy.
Actually, we know via the virial theorem that the average
potential energy should be −2 times the kinetic energy, for any
1/r potential. (This works for gravity, too.) Therefore the
number in item (2d) is only an estimate. The actual electrostatic
energy is two rydbergs, i.e. −27.2 eV. This tells us that in
some appropriately-weighted average sense, the average
electron-proton distance must be slightly larger than half an
Ånstrom, but only very slightly. Half an Ånstrom is still
a rather good estimate.
- Question 3: Why isn’t the hydrogen diameter one Fermi instead of one
Ångstrom? Why doesn’t the electron just spiral in and sit on
top of the proton? It would have 105 times more binding energy
there. Answer: If you try to confine an electron to such a small
volume, it’s kinetic energy would go up. Conceptually (and even
semi-quantitatively) you can think of the hydrogen atom as an
electron in a “container” with electrostatic forces pushing in
from all sides. The electron-proton electrostatic attraction is
like the anvil in figure 1, trying to make the
container smaller, but it is opposed by the pressure of the
electron-gas inside the container.
- Question 4: In figure 1, if we lower the temperature of
the gas, it shrinks. At zero temperature the anvil goes all the
way to the bottom. Does question 3 imply that the size of the
hydrogen atom should be proportional to temperature, too?
Answer: No. The kinetic energy of the atomic electron
is essentially independent of temperature until you get
to very high temperatures. At normal temperatures and below,
the KE “levels off” at a value that depends only on the size of
the box. This has got nothing to
do with the electron’s charge; neutral particles such
as neutrons and even photons behave the same way.
- Question 5: How can we understand this “leveling off”
of the low-temperature pressure in terms of high-school
Newtonian mechanics?
Answer: We can’t. We don’t live in a Newtonian
universe. We live in a universe governed by the laws of
quantum mechanics. Get used to it.
- Question 6: But how come we used one set of rules for question 0 and
another set of rules for question 4?
Answer: We didn’t. Quantum mechanics gives the right answer for
question 0 and question 4. QM always gives the right
answer. Sometimes the classical approximation also gives
the right answer, but that is just icing on the cake. In question
0, the compression of the gas can very accurately be described
quantum-mechanically, in terms of shortening the wavelength of the
wavefunction of the gas particles in proportion to the shortening
of the region they’re in. See section 5 for
details.
-
You can demonstrate this very clearly with a gas of
microwave photons. Start out with one liter of
microwaves at 1 GHz and compress them down to half a
liter at 2 GHz.
- You can demonstrate the key idea using
a violin: Start with your finger near the nut, say A# on
the A string. Pluck the string. Then slide your
finger to shorten the active part of the string by a
factor of two. You’ll hear the frequency go up an
octave. The total energy of the vibrations will be
increased, because you did work on them as you
compressed them into the smaller region.
Remember: The quantum description is always right. The
classical description is an approximation that is
sometimes valid at high temperatures and low densities
(and not necessarily even then). If you take any
approximate law and extrapolate it beyond its range of
validity, you’ll get fooled for sure.
- Question 7a: How come you never told me this before?
Answer: You didn’t ask. If you do experiments in the
regime where the classical approximation is valid, it’s
hard to notice non-classical effects.
- Question 7b: Why can’t you give me a simple classical explanation of
degeneracy?
Answer: You’re specifically asking about a non-classical
phenomenon. That’s what degeneracy means. So don’t be surprised
if you get a non-classical explanation. If you’re allergic to
bread and cheese and tomatoes, don’t order a pizza.
- Question 8: Why is a sodium atom markedly larger than a neon atom,
and why is its first ionization energy markedly lower?
Answer: Electrons are fermions. That makes their
wavefunction slightly different from waves on a violin
string. On a violin, you can excite the fundamental
mode as much or as little as you want, over a very wide
range. For electrons, you can’t do that. You can put
at most two electrons in the fundamental mode (one spin up, one
spin down) and at most two in the first partial, and at
most two in the second partial, and so on. If you want
to put a lot of electrons in a small box, you will have
to occupy some pretty high-numbered modes. These have
lots of momentum, so the zero-temperature pressure is
higher than it would be for non-fermions. The valence
electron in a sodium atom has so much kinetic energy
that the nucleus can barely hold on to it.
You won’t notice this at high temperatures and low
densities, because then you’re trying to put, say,
hundreds of fermions into millions of modes, so you can
always find an available unfilled mode for whatever
you’re trying to do.
- Question 9: What’s this got to do with neutron stars?
Answer: The analogy between neutron stars and atoms
is rather tight. In one case, the
central force is gravitational; in the other case it’s
electrostatic. In one case the fermions are neutrons,
in the other case they’re electrons. But the essential
part of the story is the same: It’s a box full of
fermions. The pressure is due to the momentum and
kinetic energy, which has to do with wavelength.
- Question 10: What does this tell us about non-metallic solids?
In a solid
object such as a sugar crystal, the atoms and molecules are
sitting pretty much cheek-by-jowl (unlike a gas where there is
plenty of room between the particles). Within each atom, the
electrons are degenerate. If you try to compress the
solid, you encounter a fair amount of degeneracy pressure.
This explains why the speed of sound is higher in solids
than in gasses.
- Question 11: What does this tell us about metals?
In a metal, you
have a crystalline lattice of metal ions, which is by
itself a solid and resists compression for the reasons
discussed in the previous item. But in addition, in
the metal you have the conduction electrons, which are
highly degenerate, and exert their own “degeneracy
pressure”.
- Question 12: What does this tell us about the fundamental forces
(gravitational, electroweak, and strong)?
Answer: Nothing. Particles have momentum and kinetic
energy quite independent of which fundamental force(s)
they interact with. And boson/fermion character is
independent of the fundamental forces. If you’re trying
put too many fermions into a mode, you can’t “force”
them in there using a stronger “force”. It’s just not
going to happen. They will either go into another mode
or not go in at all.
- Question 13: What if we redefine “kinetic” energy?
Oddly enough, that is possible. The definition of “kinetic”
energy depends on what you take as “the” coordinate(s). Note the
contrast:
|
If you look at an ideal gas microscopically, all the energy
is KE.
|
|
If you put the gas in a cylinder and take the macroscopic
position of the piston as the only coordinate of interest, the gas
is like a spring,1 and there’s a potential energy ... in this coordinate
system.
|
In classical canonical mechanics, there’s a formal symmetry. You can
perform a change of coordinates that turns position into momentum, and
momentum into position. That is, you can turn KE into PE and vice
versa.
Be that as it may, if we stick to the usual coordinates for electrons
and protons, the picture presented in this section is correct: the
microscopic kinetic energy is the only thing that prevents atomic
electrons from spiraling into the nucleus.
Bottom line: There’s nothing special about “degeneracy” pressure.
It’s just pressure. The pressure in a degenerate Fermi fluid is
higher than it would be in a non-degenerate or non-fermionic fluid
under comparable conditions of density and temperature ... but it’s
just a difference in quantity, not a difference in kind. The most
remarkable thing is that the pressure doesn’t go to zero at low
temperatures.
2 Annihilation, not merely a Node
What we know about interference patterns is partially but not entirely
helpful in explaining the annihilation of the wave function when two
fermions try to get into the same state. Let’s compare the two ideas:
In the left column we discuss an interference pattern, and in the
right column we discuss exclusion:
|
Start with the pattern
which has a node whenever x is a half-integer.
It has a magnitude of 0.13 at the anti-nodes.
|
|
Start with the pattern
which has no nodes, no anti-nodes, no wavelength, no phase, and zero
amplitude. It’s no wave at all. It’s the unwave.
|
|
An interference pattern is produced by the overlap of two waves with
different wavevectors. The spacing of the interference fringes
depends on the difference between the two wavevectors.
|
|
The exclusion
principle applies to two fermions with the same wavevector (and same
spin label et cetera). There won’t be an interference pattern. There
won’t be nodes. There will be no wave at all.
|
In a many-body problem there is such a thing as the vacuum state,
representing the state where there are zero particles. Call it
|vac⟩. It’s a perfectly good state. Now, if you apply
the same creation operator twice (starting from that state or any
other state) the result is not |vac⟩, the result is
nothing at all. The unstate. Zilch. Not |vac⟩ but
0.0 |vac⟩, which is not at all the same.
3 Classifying Interactions as “Fundamental” Or Not
Note: It is preferable to speak of fundamental interactions rather than
fundamental forces.
The current canonical list of fundamental interactions is:
-
The electroweak interaction, which comprises
-
electric fields,
- magnetic fields, and
- the weak nuclear interaction.
- The strong nuclear interaction.
- Gravitation.
At present we have no good way to explain any of these interactions in
terms of the others, nor to explain them in terms of some
more-fundamental description – although efforts continue in this
direction. So for now at least, these are considered distinct and
equally fundamental.
The list changes from time to time. In the middle of the 19th
century, electrostatics and magnetostatics were considered distinct
and fundamental. Later they came to be seen to be special cases of
the unified, more-fundamental electromagnetic interaction, which
claimed a place on the top-level list, demoting electricity and
magnetism to sub-items. Similarly, when the weak nuclear interaction
was first discovered, it was considered distinct and fundamental, but
later it (along with electromagnetism) was seen to be a special case
of the more-fundamental electroweak interaction, which claimed a place
on the top-level list, demoting the weak interaction to a sub-item.
Pressure might have been on the list in the early 19th century, but it
was stricken when people realized it can be fully explained in terms
of the other items on the list, via “kinetic theory”. The idea of
degeneracy arose much later, so there was never the slightest chance
that the pressure of a degenerate gas would be considered a
fundamental interaction.
The gravitational interaction is a field. It has dimensions of force
per unit mass. Pressure is also a field. It has dimensions of force
per unit area, or (equivalently) energy per unit volume. The question
is not whether there’s a force due to pressure; the question is
whether it’s fundamental. It’s not. It’s merely a consequence of the
known fundamental laws.
By way of analogy: skin-friction drag on an airplane is also a force.
No doubt about it. But it’s not fundamental. It’s merely a
consequence of the known fundamental laws.
4 Gravity “Overcoming” Pressure, Or Not
One sometimes sees statements such as “a neutron star doesn’t collapse
because gravity cannot overcome the degeneracy pressure”.
That’s true except when it’s not true. It is highly overstated, and
highly open to misinterpretation.
By way of analogy: There are some books resting on my coffee-table.
You might say they don’t fall to the floor because gravity “cannot”
overcome the upward pressure that the coffee-table exerts on the
books. But:
-
“Cannot” is too strong a word, creating an
overstatement that is not entirely true.
- If/when gravity does not overcome the pressure, it is just a
consequence of the prosaic laws of physics – it is not a new 11th
commandment that supersedes the other laws of physics.
If I pile more and more books onto the coffee-table, eventually the
table-legs will buckle, and the whole pile will collapse onto the
floor.
The story for neutron stars is entirely analogous: Gravity does not
overcome the pressure of the neutron gas, except when it does. If you
start with a neutron star that is stable against collapse, and pile
more and more material onto it, eventually it will collapse. There is
no such thing as degeneracy pressure; there is just pressure. It is
just a consequence of the prosaic laws of physics – it is not a new
11th commandment that supersedes the other laws of physics.
5 Gas Pressure
Let’s calculate the pressure in a gas. It’s easy. A more-detailed
calculation can be found in reference 1, but we can
sketch the outline here. We start with the definition of pressure,
where E is the energy, V is the volume, and S is the entropy.
For a definition of entropy, see reference 2.
We begin by (temporarily) assuming that when we change the volume, the
exact same quantum states are occupied before and after the change.
For details on this, see section 8. This is more than
sufficient to guarantee constant entropy.
Consider a particle in a cubical box of size L so that V = L3.
The lowest-lying state has wavevector k = [1,1,1] π / L. A
generic state has wavevector k = [nx, ny, nz] π / L, where
1+nx is the number of nodes in the wavefunction in the X-direction.
The state has momentum p = h k / 2π. For a nonrelativistic
particle of mass m, the kinetic energy is KE = p2/2m.
The energy of the gas as a whole is
where the sum runs over all occupied states. We can write this as
where α depends on which states are occupied. We could do the
sum to obtain an exact expression for α, but that would just be
a distraction from the essential L-dependence of equation 4. For present purposes, all that matters is that α
is independent of V and independent of changes in V, assuming
gradual changes (of the kind defined in section 8). Then
we have
Hence
where γ is called the adiabatic exponent. It is
sometimes called the compressibility index but that’s a
misnomer; we would be better off calling it the stiffness index
since large γ means the gas is hard to compress. (It is also
called the ratio of specific heats for reasons that need not
concern us here.) We have calculated that γ = 5/3 for a gas of
nonrelativistic pointlike particles.
Note that γ does not depend on whether the gas is degenerate or
not! The calculation is valid for a non-degenerate non-Fermi gas such
as helium, and equally valid for a degenerate Fermi gas such as the
electrons in a white dwarf, or the neutrons in a neutron star.
For a gas of relativistic pointlike particles, the kinetic
energy is KE = pc so there is only one factor of L in the
denominator of equation 4. Therefore the energy goes like
V−1/3 and γ = 4/3.
It is evident from equation 4 that for a nonrelativistic
gas, the energy (and hence pressure) depend inversely on the mass of
the particles. A gas of N electrons in a box of size L will have
a much higher pressure than the same number of neutrons in a
same-sized box. The neutron pressure in a neutron star is higher than
the electron pressure in a white dwarf – but that is in spite of, not
because of, the mass of the particles; it is because the particles
have been stuffed into a box that is orders of magnitude smaller,
roughly 10 kilometers rather than thousands of kilometers.
6 Collapse
Consider the following analogy:
|
Build a card-house on a table-top. It is stable – not very
stable, but stable. You can quantify the stability; one component
is
where E is the energy and x is a displacement you apply to some point
on the card-house.
|
|
Similarly, consider a neutron star that is
stable. You can quantify the stability in the usual way;
one component is:
where E is the energy and x is some applied perturbation. For
instance, x could be the amplitude of a spherical sound wave (a
pulsation or
“breathing” mode).
|
|
Next, gently and smoothly jack up one side of the table, so
it tilts by an angle theta. Measure σ as a function of theta. It
will go smoothly toward zero. When σ gets too near zero, the
card-house will collapse.
|
|
Next, smoothly and gently add some mass
to the star. Measure σ as a function of mass. It will go smoothly
toward zero. When σ gets too near zero, the star will
collapse. We do not need to discuss gravitational singularities
in order to describe this process. At the time gravity
first overcomes the pressure and collapse begins,
there is no singularity.
|
To understand how a white dwarf turns into a neutron star we need to
rescind the assumption discussed in section 8. That is, we
rescind the assumption that compression results in corresponding
states. At some point, when the gravitational force is large enough,
the easiest way to compress the star is to get rid of some electrons,
combining them with protons to make neutrons. (Neutrinos are
produced, too, but they escape.) Once this process starts, the star
contracts, with a really small γ. The gravity is everywhere
stronger, with no countervailing increase in pressure, so the process
accelerates. Boom. Supernova.
Pulsations are unstable if the increase in gravity (due to an increase
in density) is greater than the increase in pressure (due to the same
increase in density). According to reference 3, for Newtonian
gravity, the criterion is γ = 4/3, so stars are stable as long
as the gas is nonrelativistic, and marginally stable even if the gas
is relativistic. However, the derivation of this result appears to
rest on some questionable approximations, and it wouldn’t surprise me
if Newtonian gravity produced instability when the gas is sufficiently
relativistic. Real gravity (general relativity) is slightly stronger
than Newtonian gravity, so at some point gravity wins.
7 Quantum Fluctuations and Thermal Fluctuations
Figure 2 shows the energy as a function of temperature of a
harmonic oscillator in thermal equilibrium. In the classical
approximation, the energy is equal to 1kT, as shown by the magenta
line. The true (i.e. quantum mechanical) result is shown by the heavy
dark line. You can see that at high temperatures, the true behavior
closely tracks the classical approximation, while at low temperatures
the true energy bottoms out at ½ℏω, which is the so-called
“zero point energy”.
The curve in figure 2 is not an artist’s conception; it is a
quantitative graph of the function
You can easily verify that this function behaves as described, by
considering the behavior of coth(x): for large x it goes
to 1, and for small x it goes like 1/x.
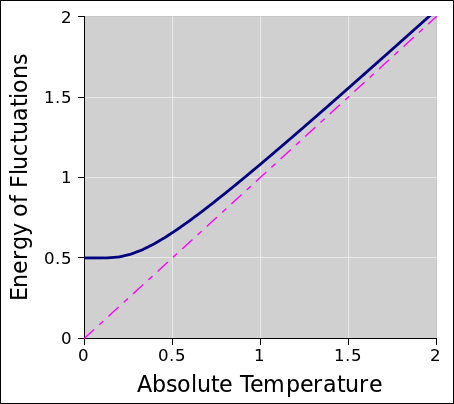 Figure 2
Figure 2: Fluctuations versus Temperature
It is worth remembering that this one function describes the thermal
fluctuations and the quantum fluctuations. Indeed, it does not
distinguish them, especially not near the “knee” in the curve in
figure 2. The notion of “purely quantum” fluctuations
and “purely thermal” fluctuations are just names we give to
the two limiting cases.
8 Appendix: Corresponding States
We have been considering a process that changes the size of the box
(L) but leaves the quantum numbers nx, ny, and nz
unchanged. The “before” state and the “after” state will not be
geometrically congruent, because the size has changed. However, the
two states will be related by a very simple topological distortion.
The wavelength will be different, but the quantum numbers nx,
ny, and nz will be the same.
You can show, using time-dependent perturbation theory, that this is
indeed how a gas behaves, provided the volume-change is gradual
enough, and no chemical reactions or nuclear reactions are taking
place.
I like to call this the corresponding-states approximation,
which is a nice descriptive name. It is sometimes called the
Born-Foch approximation. You can call it simply the
gradual approximation, if you assume there are no chemical
reactions or nuclear reactions taking place.
More commonly it is called the adiabatic approximation.
However, that is in some ways a trap for the unwary, because it
conflicts with other, older, and more-common definitions of the word
“adiabatic”.
Important note: The formulas that we have derived give a correct
description of what happens for any isentropic process, whether
it is gradual or not – but we haven’t actually proved that.
* References
-
-
John Denker,
“The Laws of Thermodynamics”
www.av8n.com/physics/thermo-laws.htm -
John Denker,
“Entropy” – Chapter 2 of Modern Thermodynamics
www.av8n.com/physics/thermo/entropy.html -
Misner, Thorne, Wheeler,
Gravitation